Industry Funded
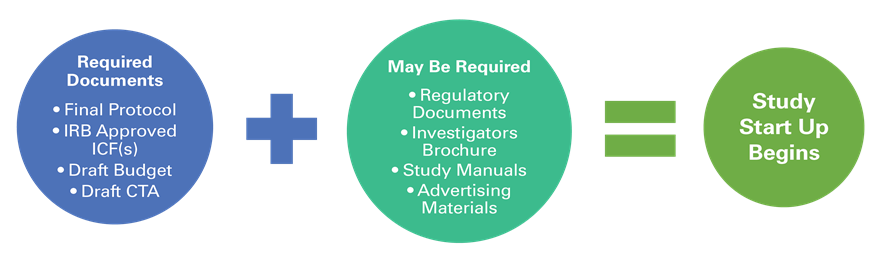
CRS determines study feasibility along with the NJH Investigator. If a study is feasible, and NJH is selected as a site, all required documents for the study must be received before work begins. Required documents will vary based on the type of study (i.e. Industry funded, FDA regulated vs. Non-Interventional, Investigator-Initiated trial). The following may be required before start-up begins:
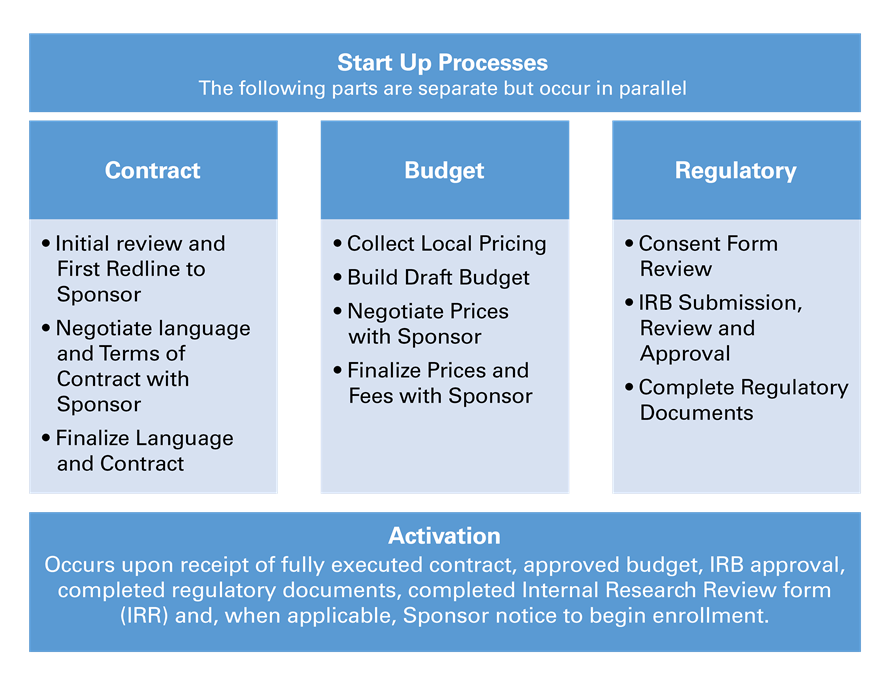
After all required documents are received, start-up processes will begin. For Industry sponsored clinical trials, CRS works directly with study Sponsor and/or Clinical Research Organization (CRO) for a smooth and streamlined start-up process. Contract, budget, regulatory and IRB processes initiate at the same time and are worked on in parallel.
Goal time to activate a new study for enrollment is 12 weeks.
Investigator-Initiated Trials (IIT)
The start-up process for IITs is different from Industry trials. IITs may or may not involve FDA regulated products and funding sources vary. Investigators at National Jewish Health take on a larger role in the start-up of IITs.
Investigators compile and submit required materials to the IRB for review and approval. When CRS services are required for a study, the Investigator works with the CRS for guidance with budget preparation. CRS provides support for the Investigator based on the needs of the study.
Activation steps for an IIT are slightly different from Industry sponsored trials. Often, the grant/award may be the first item finished which means the IRB and budget processes are finalized subsequent to award receipt. Sometimes, proof of IRB submission is required before a grant can be submitted. After the study has IRB approval, and budget and grant/award are finalized, the study may proceed. An IRR is still required.
The Clinical Research Coordinator requires time to learn the protocol, create source documents, and prepare for the study. If CRS support is required and the sooner CRS is involved, the more time we’ll have to prepare before enrollment is ready to begin.